Myofascial Pain Syndrome
by Mike Petty, DVM Download the slides here Dr. Mike Petty hosts this comprehensive webinar on one ...
Watch NowPhotobiomodulation therapy is defined as a form of light therapy that utilizes non-ionizing light sources, including lasers, light-emitting diodes, and/or broadband light, in the visible (400 – 700 nm) and near-infrared (700 – 1100 nm) electromagnetic spectrum. It is a nonthermal process involving endogenous chromophores eliciting photophysical (i.e., linear and nonlinear) and photochemical events at various biological scales. This process results in beneficial therapeutic outcomes including (but not limited to) the alleviation of pain or inflammation, immunomodulation, and promotion of wound healing and tissue regeneration.1 The term photobiomodulation (PBM) therapy is now being used by researchers and practitioners instead of terms such as low-level laser therapy (LLLT), cold laser, or laser therapy.
The fundamental principles that underpin photobiomodulation (PBM) therapy, as currently understood in the scientific literature, are relatively straightforward. There is consensus that the application of a therapeutic dose of light to impaired or dysfunctional tissue leads to a cellular response mediated by mitochondrial mechanisms that reduce pain and inflammation and speed healing.
The primary target (chromophore) for the process is the cytochrome c complex, which is found in the inner membrane of the cell mitochondria. Cytochrome c is a vital component of the electron transport chain that drives cellular metabolism. As light is absorbed, cytochrome c is stimulated, leading to increased production of adenosine triphosphate (ATP), the molecule that facilitates energy transfer within the cell. In addition to ATP, laser stimulation also produces free nitric oxide and reactive oxygen species. Nitric oxide is a powerful vasodilator and an important cellular signaling molecule involved in many physiological processes. Reactive oxygen species have been shown to affect many important physiological signaling pathways including the inflammatory response. In concert, the production of these signaling molecules has been shown to induce growth factor production, increase cell proliferation and motility, and promote extracellular matrix deposition and pro-survival pathways. Outside the cell, nitric oxide signaling drives vasodilation, which improves microcirculation in the damaged tissue to deliver oxygen, vital sugars, proteins, and salts while removing wastes.
For PBM to occur, light needs to reach the mitochondria of the damaged target tissue. Laser therapy is applied to the surface of the skin. The best clinical results are achieved when a sufficient amount of light (number of photons) reaches the target tissue. There are a number of factors that can help maximize the light that reaches the target tissue. These include
Proper wavelength selection
Sufficient laser power
Seducing reflections
Minimizing absorption by molecules not involved in photobiomodulation
laser light has a specific wavelength, as opposed to white light, which contains a broad range of wavelengths. The unit used to measure wavelength is nanometer (nm). Much research has been done to investigate how melanin, blood, fat, and water absorb light. This has led researchers to define a window or range of wavelengths through which light can penetrate biological tissue. This window is referred to as the optical or therapeutic window.
Typically plots of the optical window are shown on a log scale, which deceptively maximizes peaks below 1 and minimizes peaks greater than 1, as shown in Figure 1. Plotting on a log scale allows for a wide range of intensities to be plotted. However, the log representation of the data can be misinterpreted; for example, looking at the absorption values from the plot, you can see that below 1000 nm the absorbance of water is less than 0.5.
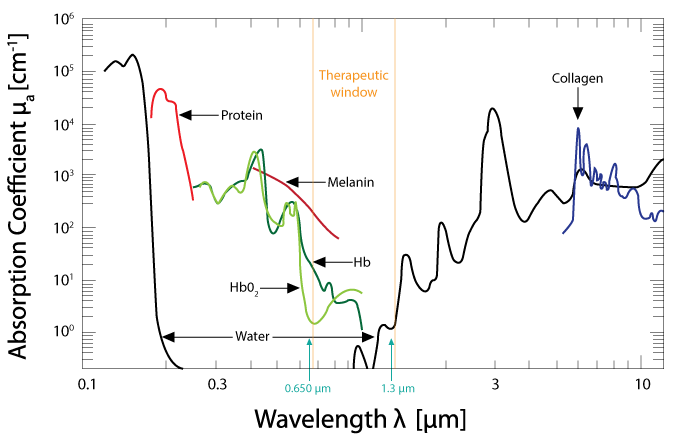
From Hamblin MR, Demidova TN. Mechanisms of low level light therapy. extends Proc. of SPIE Photonics. 2006; 6140: 614001-01-12. doi: 10.1117/12.646294
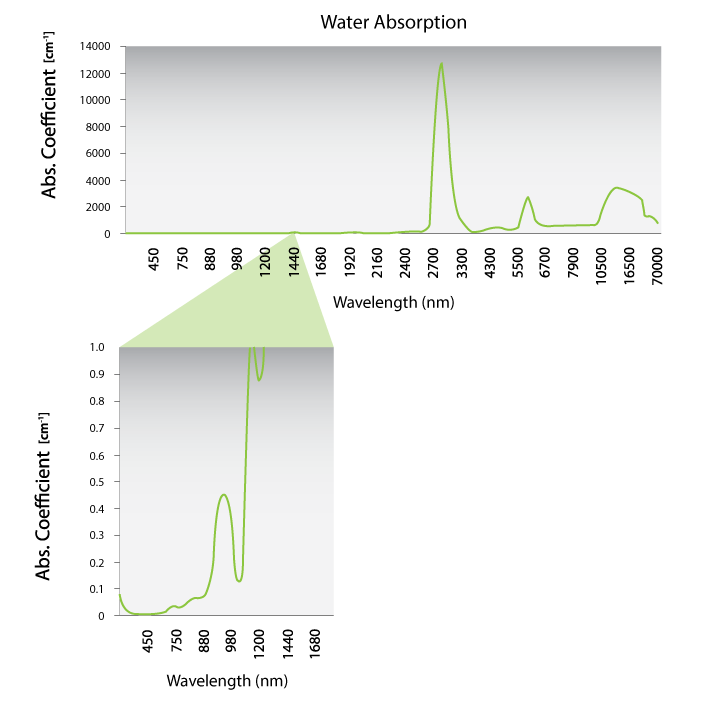
Data generated from Hale and Querry. Optical constants of water in the 200 nm to 200 μm wavelength region. Appl. Opt. 1973; 12: 555-563.
The differences in the water absorption are better viewed on a linear plot. Figure 2 shows a plot of the water absorption on a linear scale. The lower plot is a magnification of the region between 450 nm and 1400 nm. There is a small peak at 980, but it goes up to a maximum of 0.4. The absorption is dominated by melanin in the skin. Both 810 nm and 980 nm are in the “optical window” where the absorption by other chromophores is minimized. Both of these wavelengths are in the therapeutic or optical window and are effective for laser therapy. Since melanin absorption dominates at lower wavelengths, 980 nm is better for maximal penetration of skin with melanin.
Companion’s CTO, Luis DeTaboada, has also done modeling of the penetration depth of the various wavelengths of laser to compare them.
Data generated from Hale and Querry. Optical constants of water in the 200 nm to 200 μm wavelength region. Appl. Opt. 1973; 12: 555-563.
The main components in the tissue that absorb light are: melanin, oxyhemoglobin, deoxyhemoglobin, fat, and water. Melanin absorbs light strongly in the lower wavelengths, so dark skin will absorb more light, especially the wavelengths from 500 nm to 800 nm. Wavelengths longer than 1200 nm are readily absorbed by water.
Lasers with these longer wavelengths are typically used in ablative procedures such as surgery or skin resurfacing. The current understanding of photobiomodulation is that light in the wavelength range of 800 nm to 1000 nm is capable of penetrating the skin and surface tissue and reaching the muscle below.
Companion lasers typically emit a blend of 810 nm and 980 nm light. For treatment selections of dark skin (and/or dark fur), the laser will automatically shift to all 980 nm light; thus minimizing the amount absorbed by melanin.
Figure 3 shows a plot of the various absorption coefficients as a function of wavelength on a linear scale. This shows the dominance of melanin absorption compared to hemoglobin, oxyhemoglobin, water and fat.
Red light (600 nm to 700 nm) can generally be used to treat the conditions near the surface of the skin, but light in the 800 nm to 1000 nm is needed to reach deeper tissue structures.
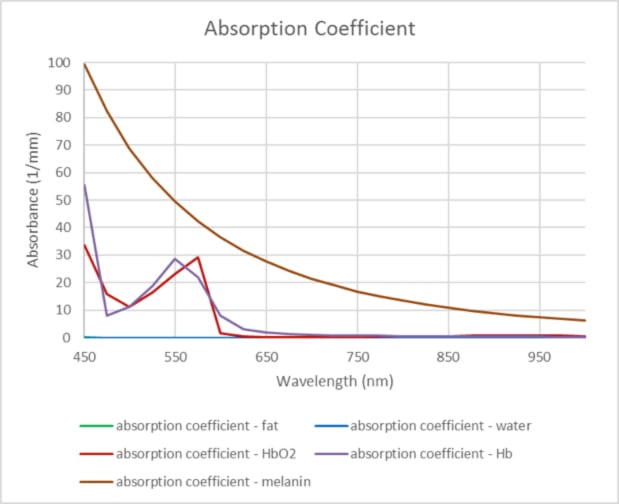
Linear plot of Absorption Coefficient vs. Wavelength. Data for the absorption coefficients were obtained from Oregon Medical Laser Center, http://omlc.org.
The laser light energy is measured by the laser power.
Power seems simple, but simply stating the output power does not relay the whole story when discussing therapy laser treatment. Not only is power important, but so is the size of the area that is being treated. Typical power units for a laser are watts (abbreviated as W). Power is a measure of the number of photons emitted from the laser each second. Early therapeutic lasers had very low powers (less than 0.5 W) and very small beam areas (or spot sizes); consequently, early studies were often disappointing because the low powers were not able to provide a sufficient number of photons to reach deeper affected tissue.
The Food and Drug Administration (FDA) classifies lasers according to their output power and recognizes four major classes (I to IV), including three subclasses (IIa, IIIa, and IIIb). In December 2003, the FDA approved the first class IV laser for the relief of minor muscle and joint pain. In October 2006, LiteCure, Companion's parent company was formed and FDA approval for the LCT-1000 a class IV medical therapy laser was granted in February 2007. Companion lasers are class IV lasers; they have an output power that is greater than 0.5 W.
Because class IV lasers have a higher output power, there are some additional safety considerations that should be followed when using a class IV laser. Eye safety is the most important consideration and the laser light should not be directed into an eye. The practitioner and patient should wear approved safety glasses for further protection from inadvertent beam reflections.
It is important to note that the Companion lasers not only have higher power, but a larger beam area. This makes them more capable of delivering a therapeutic dose to larger treatment areas.
Simply stated, the greater the number of photons delivered to the surface, the greater the number of photons at any tissue depth. There is a threshold—a minimum number of photons that are needed to “turn on” the therapeutic effects of laser light. Hundreds of scientific studies have been done in vitro and have characterized the dosages needed to achieve a cellular response with light. These studies provide a baseline for the amount of laser energy needed to achieve results at the cellular level. PBM therapy is non-invasive; the light is applied to the surface of the skin. Some of that light is reflected by the skin or absorbed by other chromophores that are not associated with the injured cells and therefore do not contribute to PBM. A sufficient dose needs to be applied to the skin so that a sufficient dose reaches the skin despite these losses and PBM occurs at the target tissue.
The greater the power, the greater the penetration to deeper tissue for a given amount of time. With the higher-powered lasers, it is possible to not only apply the benefits of PBM superficially, but it is also possible to treat a greatly expanded range of conditions by delivering a clinically effective quantity of photons to cells deep within the tissue.

The figure illustrates, with infrared images, the amount of light seen on the back of the hand when laser light is applied to the palm at 1 watt, 5 watts, and 10 watts of power.
Companion lasers are not designed for spot treatment. Instead, the treatment area is larger than the beam area and the PBM treatment is delivered by scanning the treatment head over the treatment area in a continuous movement. Treatment with Companion lasers is non-invasive, and it is not possible to measure the dose delivered to the target tissue; it is only possible to measure the dose delivered to the treatment area.
However, we do know from cadavers and live animal studies and modeling that the absorption of the laser in the skin and fat layers on its way to the target tissue can significantly reduce the dose that gets to the target tissue. The most prevalent method of indicating laser therapy dosage is to measure the density of energy applied to the tissue surface. This is typically expressed in J/cm2. Some variation in clinical effects can be observed; particularly at very high (>50W) or very low (<1 W) power settings using the same J/cm2 dose. The measurement technique works well for typical treatment protocols but is not absolutely ideal. Companion Animal Health provides a treatment guide with each device with recommended dosing for different clinical conditions.
Therapy lasers can pulse in different ways. Generally speaking, therapy lasers that pulse either operate in a superpulsed mode or a gated mode. In superpulsed mode, a large amount of energy is allowed to build up over a period of time and then released quickly in a single, large burst of energy. Superpulsed lasers produce very brief pulses (on the order of 200 nanoseconds) of high frequency. The pulses have high peak powers (1-50 W) with a much lower average power (such as 60 mW).
Photobiomodulation therapy can be delivered in either continuous wave (CW) or pulsed modes. As photobiomodulation therapy has become more popular, manufacturers have made elaborate claims about specific pulsing protocols and application-specific wavelengths. These claims can be misleading and can create confusion. In reality, these product-specific bells and whistles are more marketing hype than science. A review by Hashmi et al. that looked at CW versus pulsed light concluded that more evidence is needed. In general, the use of pulsing decreases light delivered to the surface and hence to the target.
Companion lasers do have the option to be operated in a pulsed mode. In the pulsing mode for Companion lasers, the laser is gated: it cycles its CW power on and off and consequently delivers a lower average output power. If you are treating a small region and would like to treat for a longer time at a lower power, switching to a pulsed mode will decrease the effective power and dose delivered to the target by 50%.

by Mike Petty, DVM Download the slides here Dr. Mike Petty hosts this comprehensive webinar on one ...
Watch Now
by João Alves , DVM, MSc, PhD, DECVSMR
Watch Now
by Becky Murray, DVM, DACVS
Watch Now